[STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN II
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
L'altro ieri Pablo Tebas ha partecipato al 14° incontro annuale dell'Institute of Human Virology (IHV) a Baltimora e ha presentato una breve relazione sul suo trial di fase I dell'SB-728-T (Results of a phase 1 using CCR5 Deficiency with Zinc Finger Nuclease-modified Autologous CD4 T Cells (SB-728-T) in HIV-infected Subjects).
Qualcuno ne sa qualcosa?
Qualcuno ne sa qualcosa?
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Non so nulla di nuovo, ma ho trovato una discussione su investor village in cui questi "investor" parlano (non so se a ragion veduta) del fatto che Sangamo non ha mai raggiunto livelli così alti di ricerca e che la "terapia 728", se i dati saranno confermati, potrebbe essere davvero una novità miliardaria per la società (e ovviamente i suoi investors).
Si parla soprattutto dei potenziali tempi di approvazione della terapia: da un lato alcuni sono giustamente cauti, invitando ad attendere i prossimi dati più consistenti, dall'altro (in classico stile americano) molti parlano di una probabile approvazione in tempi rapidi nel momento in cui la terapia 728 venisse presentata come MIGLIORATIVA della HAART attuale (in termini di cura ed effetti collaterali), lotta che, sostengono gli investors, sarà sicuramente appoggiata dai gruppi di sieropositivi americani, permettendo un rapido ingresso sul mercato. E quindi soldoni.
In ogni caso, se tutto ciò si verificasse (incrocio di dita), i primi a beneficiarne sarebbero sicuramente gli americani (più facoltosi). Di seguito l'immagine dello stato degli studi di Sangamo su ZFN

Si parla soprattutto dei potenziali tempi di approvazione della terapia: da un lato alcuni sono giustamente cauti, invitando ad attendere i prossimi dati più consistenti, dall'altro (in classico stile americano) molti parlano di una probabile approvazione in tempi rapidi nel momento in cui la terapia 728 venisse presentata come MIGLIORATIVA della HAART attuale (in termini di cura ed effetti collaterali), lotta che, sostengono gli investors, sarà sicuramente appoggiata dai gruppi di sieropositivi americani, permettendo un rapido ingresso sul mercato. E quindi soldoni.
In ogni caso, se tutto ciò si verificasse (incrocio di dita), i primi a beneficiarne sarebbero sicuramente gli americani (più facoltosi). Di seguito l'immagine dello stato degli studi di Sangamo su ZFN

Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Ieri la solita conference call per gli investitori (trascrizione seekingalpha.com).
L'unica cosa di rilievo raccontata da Lanphier, a parte la conferma che i dati preliminari dei trial di fase II saranno disponibili a metà 2013 e i dati completi alla fine dell'anno prossimo, è che Sangamo sta continuando ad analizzare i dati di fase I e sta conducendo un follow-up a lungo termine sulle persone che hano partecipato a quegli studi.
L'unica cosa di rilievo raccontata da Lanphier, a parte la conferma che i dati preliminari dei trial di fase II saranno disponibili a metà 2013 e i dati completi alla fine dell'anno prossimo, è che Sangamo sta continuando ad analizzare i dati di fase I e sta conducendo un follow-up a lungo termine sulle persone che hano partecipato a quegli studi.
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Fermo restando il fatto che Lanphier ha promesso che Sangamo presenterà i dati delle sperimentazioni di fase II dell'SB-728-T al prossimo CROI e che quindi di novità al momento non ce ne sono, è appena uscito su Seeking Alpha un lungo articolo dedicato alle nucleasi a dita di zinco e alle TALEN (transcription activator-like endonucleases), la tecnologia che sta entrando in competizione con le ZFN per la modificazione genetica di cellule come i CD4 e le staminali. Poiché di TALEN ancora non ci siamo occupati, anche se l'interesse di questo articolo è finanziario, più che scientifico, mi sembra valga comunque la pena di segnalarlo.
Long Opportunity Emerges As Sangamo Leverages Gene Editing To Hemophilia, Huntington's, Other Genetic Diseases
November 27, 2012
Rex Graham (CEO di San Diego Biotechnology Connection)
The odds of a major advance in the fight against HIV/AIDS have improved with the recent announcement by Sangamo Biosciences (SGMO) that its gene therapy treatment has achieved a "functional cure" for some HIV patients in its Phase 1 clinical trials.
Tim Brown, the famous "Berlin patient" and first person cured of HIV, appeared at the HIV Cure Forum in Palm Springs and West Hollywood, Calif., on Nov. 13 and 14. Brown's visit and Sangamo's clinical trial results draw attention to an unlikely corner of human gene therapy: beneficial mutations. In Sangamo's case, its scientists generate mutations in the CCR5 gene in human CD4 T cells that conferred resistance to HIV-1, the most common strain of the virus. Brown was cured when he received donated CD4 T cells with a naturally occurring CCR5 mutation.
The Richmond, Calif.-based Sangamo has touted results at the one-year clinical trial endpoint: in five of nine subjects, CD4 T cell counts persisted a year after infusion at greater than 500 cells/mm3, the accepted threshold for initiation of HAART therapy.
"The initial results in a few patients look good and compelling," said Liana Moussatos, an analyst with Wedbush Securities. "I'd like to see the same things in Phase 2 trials with more patients. Also, I'm interested in maintenance after treatment with modified CD4 cells: how durable are those cells over time?" (The number of genetically modified CD4 T cells slowly declines over time.)
Sangamo's hoped-for "functional cure" for HIV is currently in two Phase 2 studies designed to maximize the engraftment of CCR5-disrupted T-cells. The two studies, which will focus on reduction or elimination of detectable virus in treated patients, are expected to have preliminary data in the first half of 2013 and final data later that year.
HAART therapy
Currently, the most effective HIV treatment, developed two decades ago, is a cocktail of drugs. Highly active anti-retroviral therapy (HAART) is so effective that it usually suppresses the virus to undetectable levels. However, HAART therapy doesn't prevent the virus from persisting indefinitely in patients' resting CD4 T cells, and reappearing when patients discontinue therapy.
Experts agree that switching the therapeutic focus from the virus to human genes, particularly the CCR5 gene, is the most promising prospect for a cure.
Engineering genetic cures
A once- or twice-a-year genetic-modification treatment like the one Sangamo is developing could make concerns over the durability of modified CD4 T cells irrelevant. It might be more acceptable to patients than drug therapy, which can cost up to $40,000 per year and cause toxic side effects.
At the heart of Sangamo's clinical trial is a class of enzymes called zinc finger nucleases (ZFNs). As their name implies, ZFNs contain zinc ions and protein "fingers," each of which binds to a specific nucleotide triplet. Four such fingers in one molecule bind to a specific 12-nucleotide sequence. An enzyme nuclease motif cleaves DNA at the targeted binding site.
Sangamo's gene-modifications are so precise and nontoxic that the company and researchers worldwide are working not only to optimize ZFN therapies for HIV, but also for hemophilia, Huntington's disease and hemoglobinopathies such as sickle cell anemia and beta-thalassemia. Edward Lanphier, president and CEO of Sangamo, said on Nov. 13 that he would provide details on the "timelines, development steps and next critical development points" on Dec. 6, 2012.
Sangamo scientists, working with colleagues in Italy, demonstrated in preclinical studies published in 2012 in Nature Medicine that ZFNs can be used to create safer and more potent cancer immunotherapies.
In 2012, Sangamo signed a collaborative agreement involving its zinc finger DNA-binding protein (ZFP) gene regulation technology to Shire AG (SHPG), under which Shire will develop and commercialize products to treat or diagnose hemophilia. The two companies also are developing a ZFP therapeutic for Huntington's disease, a now-incurable inherited neurodegenerative disease, and Shire also is able, under the agreement, to develop treatments for two additional gene targets. Sangamo is responsible for all research activities through the submission of an Investigative New Drug Application (IND) or European Clinical Trial Application (CTA) for seven DNA targets and Shire will pay Sangamo $8.5 million per target at completion of each IND.
Sangamo received a $13 million up-front payment from Shire, and Sangamo is eligible to receive up to $213.5 million for each of seven DNA sequences targeted; Sangamo also is eligible to receive a tiered double-digit percentage of net sales of commercialized products. No timetable has been set for the collaboration with Shire.
Sangamo will update analysts about its 2013 clinical plans on Dec. 6, 2012. In the meantime, income from collaborative agreements rose from $6.1 million in fiscal 2011 to an estimated $15 million in fiscal 2012, with total operating expenses falling from $46 million to $44.5 million over the same time period. The company's net loss will fall from $35.7 million in fiscal 2011 to an estimated $25.3 million in fiscal 2012.
(...)
Elusive viral target
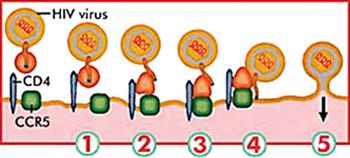
HIV-1, the most common strain of the virus, enters human T cells by binding simultaneously to two cell-surface receptors made by the CD4 and CCR5 genes. (Other HIV strains bind to the CD4 CXCR4 receptors.) Approaches that have blocked viral binding have cured other viral diseases, but not HIV.
Vaccines targeting the HIV-1 envelope gp120 glycoprotein, the viral coat protein that binds to human T cells, have been ineffective because the viral gp120 is chameleon like: vaccines against one version of gp120 usually don't work against others. In addition, anti-viral drugs intended to block gp120 binding to T cells lose their potency for similar reasons.
First cure for HIV
The first HIV cure was partly an act of desperation, but it opened the door to the genetic-mutation approach to thwart the disease.
Brown, suffering from cancer and dangerously low CD4 T cell counts in 2007, was given a bone marrow transplant from a donor who was homozygous for a mutant form of the CCR5 gene. The transplant helped cure the cancer and made Brown completely resistant to HIV-1. Brown's health improved dramatically; he discontinued anti-viral drug therapy and the virus has not reappeared in his blood.
Bone marrow transplants from unrelated donors like Brown's are impractical and can cause life-threatening complications such as graft-versus-host disease. Sangamo's gentler approach involves withdrawing about one billion CD4 T cells from each patient, and using ZFNs to mutate one or both of the copies of the CCR5 gene in each cell. One of the two CCR5 genes is mutated in 30-40 percent of the cells; both CCR5 genes are mutated in 5-10 percent of the cells. After the ZFN treatment, the cells are expanded to about 10 billion and they returned to the patient, after which the treated cells are believed to continue expanding in number.
Off-target questions
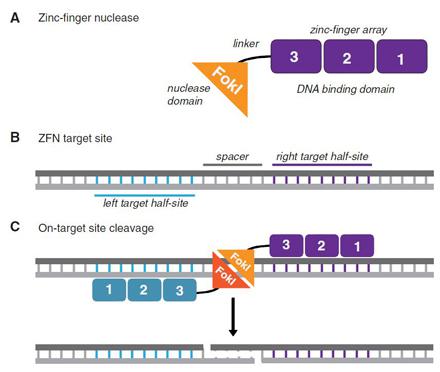
When ZFNs get inside a cell, they can cause off-target gene cleavages, which could be toxic. To diminish off-target effects, the specificity of ZFNs has been greatly improved in Sangamo's SB-728-T therapy:
The DNA break made by the FokI dimer automatically recruits the cell's error-prone repair mechanism -- non-homologous end-joining. In this process, one nucleotide is often incorrectly added or deleted, causing a shift in the CCR5 gene's "reading frame" that is so catastrophic that the gene is "knocked out." The treated, HIV-resistant cells are re-infused back into each patient, and researchers hope that cell division will yield more HIV-resistant CD4 T cells.
Zinc-finger competitor
ZFNs have a competitor in the form of transcription activator-like endonucleases (TALENs). Discovered in 2007 by plant-pathogen researchers, TALENs are virulence factors made by Xanthomonas, a genus of proteobacteria that causes leaf spots, streaks and other plant injuries. The pathogen's TALENs bind to the host plant's gene-promoter sequences to up- or down-regulate genes in ways that allow the pathogen to flourish.
"It's amazing that this underfunded area of plant physiology has created this promising technology that could be used to treat and potentially cure human diseases," said Adam Bogdanove, a discoverer of TALENs while at Iowa State University and now a professor of plant pathology and plant-microbe biology at Cornell University.
Bogdanove and colleagues at the University of Minnesota licensed their discoveries to Cellectis, a French company, in January 2012. Other TALENs intellectual property (IP) generated by plant biologists from Martin Luther University in Halle, Germany, is now owned by the Two Blades Foundation, based in Evanston, Ill. Two Blades has sold an exclusive license to Carlsbad, Calif.-based Life Technologies (LIFE). Both Cellectis and Life Technologies are aggressively marketing custom, gene-specific TALENs to researchers worldwide.
Sangamo has guarded the intellectual property related to virtually all the major ZFN discoveries, and has licensed the technology to St. Louis, Mo.-based Sigma-Aldrich (SIAL), which develops and markets tailor-made ZFN kits to researchers.
In 2011, Sigma was selling its ZFN kits for as high as $35,000 each to biotech companies and $25,000 each to academic institutions. However, those prices have plummeted to as low as $3,999 per kit due to competition from TALEN kits selling for $5,000 each.
Switching to TALENs
TALENs and ZFNs can be designed to use the same FokI endonuclease, but TALENs use simpler rules for sequence-specific DNA recognition, requiring less optimization than ZFNs.
"Lots of people are switching from zinc fingers over to the TALENs," Dana Carroll, a biochemistry professor at the University of Utah and an expert on both TALENs and ZFNs, said in a telephone interview. "I think things are moving very rapidly toward TALENs instead of ZFNs, and Sangamo is moving that way, too."
TALENs have had a major disadvantage: their size - they are much larger than ZFNs and therefore more difficult to deliver into a target cell.
The industry leader in ZFNs, Sangamo has also embraced TALENs. Sangamo researchers have published a recent study documenting that reducing the size of TALENs produced the desired gene disruptions at higher frequencies. The 2011 paper in Nature Biotechnology by researchers at Sangamo and Université de Nantes in France reported that truncated TALENs can be created to knock out genes with high selectivity in the rat, an important model for many human diseases.
Sangamo also has licensed ZFN technology to Dow AgroSciences (DOW) for the development of genetically improved maize, canola and other crops. (Dow pays Sangamo 25 percent of all ZFN-related sublicensing revenue it receives.) Rather than add bacterial or other foreign genes to a crop genome, ZFNs allow Dow researchers to modify a plant's existing genome, a feature that could prompt fewer objections from consumer activists and watchdog groups that are opposed to all genetically modified crops.
Since both ZFNs and TALENs use the same FokI endonuclease, the issue of off-target mutagenic effects is a fear in both cases. The technology is maturing quickly, and Sangamo has resolved the off-target issue.
"Sangamo is not trying to take shortcuts: it is doing things that nobody else can do," Moussatos said.
For example, Sangamo researchers, collaborating with scientists at MIT and the Whitehead Institute for Biomedical Research, both in Cambridge, Mass., described in a 2011 paper in Nature Biotechnology how the researchers combined two TALENs that each recognized specific 17-nucleotide sequences. The two TALENS both carry FokI domains that make double-stranded breaks in human embryonic stem cells and induced pluripotent stem cells.
The researchers assessed the frequency with which one TALEN pair makes a specific double-stranded break at five locations. The research team focused on 20 non-target nucleotide sequences that are most similar to the five intended targets.
Five targeted gene modifications in two genes were made at high rates: 67-100 percent; 2-24 percent; 50 percent; 1-13 percent; and 19-23 percent. (The rates were similar to those obtained with ZFNs.) Of the 20 most likely off-target hits, 18 had no off-target disruptions, one site was disrupted at a 169-fold lower rate than the intended target, and another off-target site was disrupted at a 1,140-fold lower rate than the intended target.
While the off-target effects were very low, specific gene-zapping TALENs can now be optimized even more to virtually eliminate off-target effects. The Nature Biotechnology paper demonstrated an approach necessary to validate the extremely high degree of specificity needed to modify genes in stem cells -- the ultimate prize for Sangamo and its partners.
Sangamo scientists and its collaborators have published a variety of papers regarding TALENs and applied for patents. However, no patents for ZFNs or TALENs have been issued by the U.S. Patent and Trademark Office. When patents are issued, there will be clarity around IP ownership.(...)
Long Opportunity Emerges As Sangamo Leverages Gene Editing To Hemophilia, Huntington's, Other Genetic Diseases
November 27, 2012
Rex Graham (CEO di San Diego Biotechnology Connection)
The odds of a major advance in the fight against HIV/AIDS have improved with the recent announcement by Sangamo Biosciences (SGMO) that its gene therapy treatment has achieved a "functional cure" for some HIV patients in its Phase 1 clinical trials.
Tim Brown, the famous "Berlin patient" and first person cured of HIV, appeared at the HIV Cure Forum in Palm Springs and West Hollywood, Calif., on Nov. 13 and 14. Brown's visit and Sangamo's clinical trial results draw attention to an unlikely corner of human gene therapy: beneficial mutations. In Sangamo's case, its scientists generate mutations in the CCR5 gene in human CD4 T cells that conferred resistance to HIV-1, the most common strain of the virus. Brown was cured when he received donated CD4 T cells with a naturally occurring CCR5 mutation.
The Richmond, Calif.-based Sangamo has touted results at the one-year clinical trial endpoint: in five of nine subjects, CD4 T cell counts persisted a year after infusion at greater than 500 cells/mm3, the accepted threshold for initiation of HAART therapy.
"The initial results in a few patients look good and compelling," said Liana Moussatos, an analyst with Wedbush Securities. "I'd like to see the same things in Phase 2 trials with more patients. Also, I'm interested in maintenance after treatment with modified CD4 cells: how durable are those cells over time?" (The number of genetically modified CD4 T cells slowly declines over time.)
Sangamo's hoped-for "functional cure" for HIV is currently in two Phase 2 studies designed to maximize the engraftment of CCR5-disrupted T-cells. The two studies, which will focus on reduction or elimination of detectable virus in treated patients, are expected to have preliminary data in the first half of 2013 and final data later that year.
HAART therapy
Currently, the most effective HIV treatment, developed two decades ago, is a cocktail of drugs. Highly active anti-retroviral therapy (HAART) is so effective that it usually suppresses the virus to undetectable levels. However, HAART therapy doesn't prevent the virus from persisting indefinitely in patients' resting CD4 T cells, and reappearing when patients discontinue therapy.
Experts agree that switching the therapeutic focus from the virus to human genes, particularly the CCR5 gene, is the most promising prospect for a cure.
Engineering genetic cures
A once- or twice-a-year genetic-modification treatment like the one Sangamo is developing could make concerns over the durability of modified CD4 T cells irrelevant. It might be more acceptable to patients than drug therapy, which can cost up to $40,000 per year and cause toxic side effects.
At the heart of Sangamo's clinical trial is a class of enzymes called zinc finger nucleases (ZFNs). As their name implies, ZFNs contain zinc ions and protein "fingers," each of which binds to a specific nucleotide triplet. Four such fingers in one molecule bind to a specific 12-nucleotide sequence. An enzyme nuclease motif cleaves DNA at the targeted binding site.
Sangamo's gene-modifications are so precise and nontoxic that the company and researchers worldwide are working not only to optimize ZFN therapies for HIV, but also for hemophilia, Huntington's disease and hemoglobinopathies such as sickle cell anemia and beta-thalassemia. Edward Lanphier, president and CEO of Sangamo, said on Nov. 13 that he would provide details on the "timelines, development steps and next critical development points" on Dec. 6, 2012.
Sangamo scientists, working with colleagues in Italy, demonstrated in preclinical studies published in 2012 in Nature Medicine that ZFNs can be used to create safer and more potent cancer immunotherapies.
In 2012, Sangamo signed a collaborative agreement involving its zinc finger DNA-binding protein (ZFP) gene regulation technology to Shire AG (SHPG), under which Shire will develop and commercialize products to treat or diagnose hemophilia. The two companies also are developing a ZFP therapeutic for Huntington's disease, a now-incurable inherited neurodegenerative disease, and Shire also is able, under the agreement, to develop treatments for two additional gene targets. Sangamo is responsible for all research activities through the submission of an Investigative New Drug Application (IND) or European Clinical Trial Application (CTA) for seven DNA targets and Shire will pay Sangamo $8.5 million per target at completion of each IND.
Sangamo received a $13 million up-front payment from Shire, and Sangamo is eligible to receive up to $213.5 million for each of seven DNA sequences targeted; Sangamo also is eligible to receive a tiered double-digit percentage of net sales of commercialized products. No timetable has been set for the collaboration with Shire.
Sangamo will update analysts about its 2013 clinical plans on Dec. 6, 2012. In the meantime, income from collaborative agreements rose from $6.1 million in fiscal 2011 to an estimated $15 million in fiscal 2012, with total operating expenses falling from $46 million to $44.5 million over the same time period. The company's net loss will fall from $35.7 million in fiscal 2011 to an estimated $25.3 million in fiscal 2012.
(...)
Elusive viral target

HIV-1, the most common strain of the virus, enters human T cells by binding simultaneously to two cell-surface receptors made by the CD4 and CCR5 genes. (Other HIV strains bind to the CD4 CXCR4 receptors.) Approaches that have blocked viral binding have cured other viral diseases, but not HIV.
Vaccines targeting the HIV-1 envelope gp120 glycoprotein, the viral coat protein that binds to human T cells, have been ineffective because the viral gp120 is chameleon like: vaccines against one version of gp120 usually don't work against others. In addition, anti-viral drugs intended to block gp120 binding to T cells lose their potency for similar reasons.
First cure for HIV
The first HIV cure was partly an act of desperation, but it opened the door to the genetic-mutation approach to thwart the disease.
Brown, suffering from cancer and dangerously low CD4 T cell counts in 2007, was given a bone marrow transplant from a donor who was homozygous for a mutant form of the CCR5 gene. The transplant helped cure the cancer and made Brown completely resistant to HIV-1. Brown's health improved dramatically; he discontinued anti-viral drug therapy and the virus has not reappeared in his blood.
Bone marrow transplants from unrelated donors like Brown's are impractical and can cause life-threatening complications such as graft-versus-host disease. Sangamo's gentler approach involves withdrawing about one billion CD4 T cells from each patient, and using ZFNs to mutate one or both of the copies of the CCR5 gene in each cell. One of the two CCR5 genes is mutated in 30-40 percent of the cells; both CCR5 genes are mutated in 5-10 percent of the cells. After the ZFN treatment, the cells are expanded to about 10 billion and they returned to the patient, after which the treated cells are believed to continue expanding in number.
Off-target questions

When ZFNs get inside a cell, they can cause off-target gene cleavages, which could be toxic. To diminish off-target effects, the specificity of ZFNs has been greatly improved in Sangamo's SB-728-T therapy:
- · Most are designed with four fingers to target 12-nucleotide sequences.
· ZFNs can be designed as pairs, with each four-finger domain binding to opposite strands of DNA, for a 24-nucleotide target, with a 15-nucleotide "spacer" between them.
· The FokI nuclease has been optimized to eliminate off-target effects: only when two FokI domains are brought together at the spacer region does the nuclease enzyme become activated and make a double-stranded DNA break.
The DNA break made by the FokI dimer automatically recruits the cell's error-prone repair mechanism -- non-homologous end-joining. In this process, one nucleotide is often incorrectly added or deleted, causing a shift in the CCR5 gene's "reading frame" that is so catastrophic that the gene is "knocked out." The treated, HIV-resistant cells are re-infused back into each patient, and researchers hope that cell division will yield more HIV-resistant CD4 T cells.
Zinc-finger competitor
ZFNs have a competitor in the form of transcription activator-like endonucleases (TALENs). Discovered in 2007 by plant-pathogen researchers, TALENs are virulence factors made by Xanthomonas, a genus of proteobacteria that causes leaf spots, streaks and other plant injuries. The pathogen's TALENs bind to the host plant's gene-promoter sequences to up- or down-regulate genes in ways that allow the pathogen to flourish.
"It's amazing that this underfunded area of plant physiology has created this promising technology that could be used to treat and potentially cure human diseases," said Adam Bogdanove, a discoverer of TALENs while at Iowa State University and now a professor of plant pathology and plant-microbe biology at Cornell University.
Bogdanove and colleagues at the University of Minnesota licensed their discoveries to Cellectis, a French company, in January 2012. Other TALENs intellectual property (IP) generated by plant biologists from Martin Luther University in Halle, Germany, is now owned by the Two Blades Foundation, based in Evanston, Ill. Two Blades has sold an exclusive license to Carlsbad, Calif.-based Life Technologies (LIFE). Both Cellectis and Life Technologies are aggressively marketing custom, gene-specific TALENs to researchers worldwide.
Sangamo has guarded the intellectual property related to virtually all the major ZFN discoveries, and has licensed the technology to St. Louis, Mo.-based Sigma-Aldrich (SIAL), which develops and markets tailor-made ZFN kits to researchers.
In 2011, Sigma was selling its ZFN kits for as high as $35,000 each to biotech companies and $25,000 each to academic institutions. However, those prices have plummeted to as low as $3,999 per kit due to competition from TALEN kits selling for $5,000 each.
Switching to TALENs
TALENs and ZFNs can be designed to use the same FokI endonuclease, but TALENs use simpler rules for sequence-specific DNA recognition, requiring less optimization than ZFNs.
"Lots of people are switching from zinc fingers over to the TALENs," Dana Carroll, a biochemistry professor at the University of Utah and an expert on both TALENs and ZFNs, said in a telephone interview. "I think things are moving very rapidly toward TALENs instead of ZFNs, and Sangamo is moving that way, too."
TALENs have had a major disadvantage: their size - they are much larger than ZFNs and therefore more difficult to deliver into a target cell.
The industry leader in ZFNs, Sangamo has also embraced TALENs. Sangamo researchers have published a recent study documenting that reducing the size of TALENs produced the desired gene disruptions at higher frequencies. The 2011 paper in Nature Biotechnology by researchers at Sangamo and Université de Nantes in France reported that truncated TALENs can be created to knock out genes with high selectivity in the rat, an important model for many human diseases.
Sangamo also has licensed ZFN technology to Dow AgroSciences (DOW) for the development of genetically improved maize, canola and other crops. (Dow pays Sangamo 25 percent of all ZFN-related sublicensing revenue it receives.) Rather than add bacterial or other foreign genes to a crop genome, ZFNs allow Dow researchers to modify a plant's existing genome, a feature that could prompt fewer objections from consumer activists and watchdog groups that are opposed to all genetically modified crops.
Since both ZFNs and TALENs use the same FokI endonuclease, the issue of off-target mutagenic effects is a fear in both cases. The technology is maturing quickly, and Sangamo has resolved the off-target issue.
"Sangamo is not trying to take shortcuts: it is doing things that nobody else can do," Moussatos said.
For example, Sangamo researchers, collaborating with scientists at MIT and the Whitehead Institute for Biomedical Research, both in Cambridge, Mass., described in a 2011 paper in Nature Biotechnology how the researchers combined two TALENs that each recognized specific 17-nucleotide sequences. The two TALENS both carry FokI domains that make double-stranded breaks in human embryonic stem cells and induced pluripotent stem cells.
The researchers assessed the frequency with which one TALEN pair makes a specific double-stranded break at five locations. The research team focused on 20 non-target nucleotide sequences that are most similar to the five intended targets.
Five targeted gene modifications in two genes were made at high rates: 67-100 percent; 2-24 percent; 50 percent; 1-13 percent; and 19-23 percent. (The rates were similar to those obtained with ZFNs.) Of the 20 most likely off-target hits, 18 had no off-target disruptions, one site was disrupted at a 169-fold lower rate than the intended target, and another off-target site was disrupted at a 1,140-fold lower rate than the intended target.
While the off-target effects were very low, specific gene-zapping TALENs can now be optimized even more to virtually eliminate off-target effects. The Nature Biotechnology paper demonstrated an approach necessary to validate the extremely high degree of specificity needed to modify genes in stem cells -- the ultimate prize for Sangamo and its partners.
Sangamo scientists and its collaborators have published a variety of papers regarding TALENs and applied for patents. However, no patents for ZFNs or TALENs have been issued by the U.S. Patent and Trademark Office. When patents are issued, there will be clarity around IP ownership.(...)
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
È da giorni, anzi settimane, che continuano a girare notizione su Sangamo.
Dal momento che venivano sempre dalle solite conferenze per gli investitori e non dicevano in sostanza nulla di nuovo (per esempio, ieri hanno avuto un rialzo in borsa del 17%; cose così), mi parevano delle sparate per fare cassa e non le ho segnalate qui. Io sto aspettando Sangamo al varco del CROI: lì dovranno tirar fuori i dati sulle viremie e non ci saranno sconti.
Adesso, però, mi è appena arrivato un alert da Seeking Alpha con un titolo che più esaltato non si può. Eccolo qui:
Sangamo BioSciences: A Potentially Revolutionary 2013
January 15, 2013
By Ivan Deryugin
In medicine, there are certain watershed moments that become an enduring part of medical history, such as the discovery of penicillin, or the eradication of smallpox. Sangamo BioSciences (SGMO) has a chance of joining the ranks of such key medical milestones, as the company is working on one of the greatest unmet needs in medicine - a functional cure for HIV and AIDS. Sangamo is set to report new clinical data regarding its development-stage HIV/AIDS treatment this year, and if all goes well, the stock will soar as the market for an effective treatment remains wide open. The company is well capitalized to continue development of its pipeline, and considering the vast market potential of its lead candidate, shares offer a quality risk/reward, even following SGMO's impressive gains in the last year. Nevertheless, a binary biotechnology event requires risk tolerance, and we include two options strategies for the risk-averse investor.
Overview
Sangamo was founded in 1995, and since inception has spent over $270 million on the development of its ZFP technology. ZFP's, known technically as zinc finger binding proteins, are proteins that recognize and bind to a person's DNA. Sangamo uses these to create ZFP transcription factors that are able to turn genes on or off. When these proteins bind to DNA, they can regulate the expression of any gene in any type of cell. In addition, Sangamo has created ZFP nucleases (also known as ZFN's) that can be used in therapeutic gene modification. Sangamo's goal is to disrupt the mechanism of HIV/AIDS (as well as other diseases), and hopefully, find a way to emerge victorious in what many see as medicine's last frontier.
Since going public in 2000, Sangamo has lost over 45% of its value, as setbacks have called into question the company's positioning and strategy. But, shares have risen around 150% in the past year, as Sangamo has renewed hopes that it can triumph in the fight against HIV/AIDS. And if all goes well in 2013, gains should continue.
SB-728-T: A New Approach to HIV/AIDS
(...)
What Does the Clinical Data Say?
SB-728-T is currently in Phase II trials, and Sangamo expects to report preliminary data in the first half of 2013, with full data by the end of the year. Sangamo reported early data for SB-728 in March 2012 (further data was reported in September 2012, more on that a bit later). Sangamo broke this trial into 2 arms: a group of 15 patients with CD4+ T-cell counts below 500, designated as Immune Non-Responders, and a group of 6 patients with cell counts above 450, designated as Immune Responders. After a month of treatment with SB-728-T, the Immune Responders group stopped taking antiretrovirals for 12 weeks. Sangamo stated that the viral loads of these 6 patients increased initially, as expected. However, 3 of 6 patients saw a 0.8 to > 2.0-log reduction in their viral loads, and one patient's viral load dropped to undetectable levels. According to Sangamo, this patient already had a CCR5Δ32 mutation. Sangamo stated that "control of HIV-RNA (suppression of VL) correlates significantly (p < 0.05) with calculated levels of circulating CD4+ T-cells that have undergone biallelic modification (i.e. modification of both copies) of the CCR5 gene. In this trial, SB-728-T showed only minor safety concerns, with the only reported symptoms being typical of those associated with infusion injections.
Sangamo reported updated Phase I data for SB-728 at the annual ICAAC (Interscience Conference on Antimicrobial Agents and Chemotherapy) in September 2012. Sangamo reported that its Phase I dose-escalation study demonstrated that SB-728-T was well-tolerated, and resulted in what the company called "significant and sustained increases in CD4+ T-cells above baseline throughout the yearlong period reported in the study." The increase in CD4+ T-cells was observed with a p-value of p < 0.038. Notably, 5 of 9 patients enrolled in the study showed CD4+ T-cell counts of greater than 500 cells/mm3, which is seen in the medical community as the threshold for starting HIV patients on highly active antiretroviral therapy (also known as HAART) (while guidelines published by the NIH suggest that even patients with cell counts above 500 begin antiretroviral therapy, they do not take into account the potential use of SB-728-T, and are based on the assumption that eventually, patients with cell counts above 500 will begin to see declines). Further analysis of the data done by Sangamo showed that proliferation of SB-728-T post-infusion was "sustained over the year-long period reported in the study with median modified circulating cell numbers measured to be 2.04-fold relative to input at 7 days, 0.96-fold at 6 months and 1.15-fold at 1 year post-infusion." Sangamo's Phase I data suggests that SB-728-T has the potential to reshape the immune systems of HIV patients, and allow them to fight back against the disease.
On the company's Q3 conference call, Sangamo's executives were pressed about this data, and what it means for the way forward. Wedbush argued that the FDA may not deem the data strong enough for approval given that SB-728-T does not completely wipe out the HIV virus. Analyst Liana Moussatos asked, "Based on the Phase I data, I thought it was very interesting about the treatment resulting in kind of normalizing the immune system in HIV infected patients where the virus had destroyed part of it. Is this, I mean in your discussion with the FDA, if SB-728-T treatment just restored the immune system and, say, reduces the strange cancers that result or the infections but maybe doesn't reduce viral load to nothing. Would that still be a viable treatment that could get approved?" Sangamo CEO Edward Lanphier and Geoff Nichol, the company's Executive Vice President of Research & Development, responded that this is a "hotly contested kind of question," stating that in the HIV/AIDS market, FDA approval has historically been based on viral load, which is the focus of the company's Phase II trials for SB-728-T, and that the company's goal is to render HAART unnecessary by keeping CD4+ T-cell counts above 500.
While SB-728-T does not destroy the HIV virus in every single patient, it has shown promising results, and 2013 will offer investors 2 different catalysts that can drive shares of Sangamo higher. The market for HIV and AIDS treatments is set to reach nearly $22 billion by 2018, therefore Sangamo has a tremendous opportunity to create meaningful value for its investors, as well as offer HIV patients a new treatment option, even if it captures only a minority of the market.
Financials & Pipeline Review
Sangamo ended Q3 2012 with $75.816 million in cash & investments (per its latest 10-Q filing), and expects to end 2012 with around $75 million in cash & investments. Sangamo's operating cash burn for the first 3 quarters of 2012 totaled $8.305 million, and even if Sangamo were to burn $10 million per quarter, the company would still have nearly 2 years of capital left to finance its operations.
While Sangamo is, unsurprisingly, unprofitable, the company does generate revenue ($4.907 million in Q3 2012, up 164.24% year-over-year, and $12.723 million for the first 3 quarters of 2012, up 128.38% from the first 3 quarters of 2011). Sangamo has licensing agreements for its technology in place with Sigma-Aldrich (SIAL), Dow Chemical's (DOW) AgroScience division, and Shire (SHPG). The company's agreement with Sigma-Aldrich involves the use of ZFP technology as a research reagent, while its agreement with Dow involves the use of ZFP technology in plant research. Sangamo's agreement with Shire, struck at the beginning of 2012, is the most crucial of these 3 agreements. Under the terms, Shire paid Sangamo $13 million upfront and received an exclusive worldwide license to ZFP technology for 4 different genes, covering blood clotting Factors VII, VIII, IX, and X. Shire also received rights to 3 other gene targets. Of the 6 pipeline programs (including SB-728-T) that Sangamo is currently working on, Shire owns ZFP technology in Huntington's disease and hemophilia.
In October, Sangamo and Shire presented the first set of pre-clinical data for their experimental treatment for Huntington's disease. The disease is cause by a mutation in a person's HTT gene, which is inherited from their parents. The HTT gene is responsible for encoding a protein also known as HTT. The mutation consists of a repeated stretch of DNA, known as a "CAG repeat." Under normal circumstances, a person's HTT gene has 10-29 of these CAG repeats. Patients with Huntington's disease, however, usually have more than 39. The more CAG repeats, the earlier the onset of symptoms, which include muscle and nerve degeneration, and loss of memory and cognitive control. Patients usually die within 10-20 years of symptom onset. Preclinical testing in animals has shown that lowering levels of HTT protein can slow, and potentially reverse the progression of Huntington's disease. Sangamo's pre-clinical data showed that the production of mutated HTT messenger RNA fell by more than 90%, all while leaving normal HTT cells and RNA untouched. The company expects to see this program in clinical trials by 2015. The companies' hemophilia program is also set to move into clinical testing.
Options Strategy, Takeover Prospects, and Conclusions
For Sangamo investors, gains or losses in 2013 will be driven by new data regarding SB-728-T. Fortunately, Sangamo has listed options, and they allow more conservative investors to mitigate some risk while preserving upside potential (prices are accurate as of the close of trading on Monday, January 14).
Sangamo Options Strategies, August 17, 2013 Expiration Date
The August 17 expiration date is used because it encapsulates the first half of 2013, thereby allowing Sangamo investors to be protected through the company's first data release of SB-728-T, as well as giving them a chance to hold through the second data release. Of the options strategies listed above, the purchase of an $8 put is the most sensible. It caps losses at under 18% and requires a move of under 19% to be profitable, easily doable should Sangamo report positive data for SB-728-T. However, the 3 options strategies listed assume that investors have also purchased Sangamo stock outright. There is also another options strategy that I would like to highlight: the strangle. This strangle, also utilizing the August 17 options, involves purchasing the $2 call for $6.40 (giving an entry point of $8.40 per share, just 2.44% above where Sangamo is currently trading), as well as the $7 put for $0.95. The net cost is $7.35 per strangle, and it offers a similar level of protection as the options strategy outlined above, but with a smaller capital investment.
While there has been nothing substantial in the way of takeover rumors regarding Sangamo, there is always a possibility that a deal will occur given the company's cheap valuation in relation to the market that it targets. Just as GlaxoSmithKline (NYSE:GSK) did with Human Genome Sciences, Shire cuold move to take full control of Sangamo's pipeline. Alternatively, Gilead Sciences (NASDAQ:GILD) could strike a deal, although it would be motivated by different reasons. Gilead has delivered enormous profits to its investors by pioneering the treatment of HIV/AIDS; since going public in 1992, Gilead has returned almost 90,000% (Gilead has also helped enhance the lives of millions of patients). However, the fact that there is no cure for HIV/AIDS, and the fact that patients need continuous antiretroviral therapy to hold it at bay means that Gilead has much more stability than its biotechnology peers. However, should Sangamo and SB-728-T remove the need for such therapy, Gilead's business could be threatened. It is not unreasonable to assume that Gilead may make an offer Sangamo cannot refuse in order to protect its business. And with a market capitalization of less than $400 million, Sangamo is digestible for Gilead, even with a size-able premium. 2013 is poised to be a revolutionary year for Sangamo BioSciences, its investors, and HIV/AIDS patients.
(...)
Dal momento che venivano sempre dalle solite conferenze per gli investitori e non dicevano in sostanza nulla di nuovo (per esempio, ieri hanno avuto un rialzo in borsa del 17%; cose così), mi parevano delle sparate per fare cassa e non le ho segnalate qui. Io sto aspettando Sangamo al varco del CROI: lì dovranno tirar fuori i dati sulle viremie e non ci saranno sconti.
Adesso, però, mi è appena arrivato un alert da Seeking Alpha con un titolo che più esaltato non si può. Eccolo qui:
Sangamo BioSciences: A Potentially Revolutionary 2013
January 15, 2013
By Ivan Deryugin
In medicine, there are certain watershed moments that become an enduring part of medical history, such as the discovery of penicillin, or the eradication of smallpox. Sangamo BioSciences (SGMO) has a chance of joining the ranks of such key medical milestones, as the company is working on one of the greatest unmet needs in medicine - a functional cure for HIV and AIDS. Sangamo is set to report new clinical data regarding its development-stage HIV/AIDS treatment this year, and if all goes well, the stock will soar as the market for an effective treatment remains wide open. The company is well capitalized to continue development of its pipeline, and considering the vast market potential of its lead candidate, shares offer a quality risk/reward, even following SGMO's impressive gains in the last year. Nevertheless, a binary biotechnology event requires risk tolerance, and we include two options strategies for the risk-averse investor.
Overview
Sangamo was founded in 1995, and since inception has spent over $270 million on the development of its ZFP technology. ZFP's, known technically as zinc finger binding proteins, are proteins that recognize and bind to a person's DNA. Sangamo uses these to create ZFP transcription factors that are able to turn genes on or off. When these proteins bind to DNA, they can regulate the expression of any gene in any type of cell. In addition, Sangamo has created ZFP nucleases (also known as ZFN's) that can be used in therapeutic gene modification. Sangamo's goal is to disrupt the mechanism of HIV/AIDS (as well as other diseases), and hopefully, find a way to emerge victorious in what many see as medicine's last frontier.
Since going public in 2000, Sangamo has lost over 45% of its value, as setbacks have called into question the company's positioning and strategy. But, shares have risen around 150% in the past year, as Sangamo has renewed hopes that it can triumph in the fight against HIV/AIDS. And if all goes well in 2013, gains should continue.
SB-728-T: A New Approach to HIV/AIDS
(...)
What Does the Clinical Data Say?
SB-728-T is currently in Phase II trials, and Sangamo expects to report preliminary data in the first half of 2013, with full data by the end of the year. Sangamo reported early data for SB-728 in March 2012 (further data was reported in September 2012, more on that a bit later). Sangamo broke this trial into 2 arms: a group of 15 patients with CD4+ T-cell counts below 500, designated as Immune Non-Responders, and a group of 6 patients with cell counts above 450, designated as Immune Responders. After a month of treatment with SB-728-T, the Immune Responders group stopped taking antiretrovirals for 12 weeks. Sangamo stated that the viral loads of these 6 patients increased initially, as expected. However, 3 of 6 patients saw a 0.8 to > 2.0-log reduction in their viral loads, and one patient's viral load dropped to undetectable levels. According to Sangamo, this patient already had a CCR5Δ32 mutation. Sangamo stated that "control of HIV-RNA (suppression of VL) correlates significantly (p < 0.05) with calculated levels of circulating CD4+ T-cells that have undergone biallelic modification (i.e. modification of both copies) of the CCR5 gene. In this trial, SB-728-T showed only minor safety concerns, with the only reported symptoms being typical of those associated with infusion injections.
Sangamo reported updated Phase I data for SB-728 at the annual ICAAC (Interscience Conference on Antimicrobial Agents and Chemotherapy) in September 2012. Sangamo reported that its Phase I dose-escalation study demonstrated that SB-728-T was well-tolerated, and resulted in what the company called "significant and sustained increases in CD4+ T-cells above baseline throughout the yearlong period reported in the study." The increase in CD4+ T-cells was observed with a p-value of p < 0.038. Notably, 5 of 9 patients enrolled in the study showed CD4+ T-cell counts of greater than 500 cells/mm3, which is seen in the medical community as the threshold for starting HIV patients on highly active antiretroviral therapy (also known as HAART) (while guidelines published by the NIH suggest that even patients with cell counts above 500 begin antiretroviral therapy, they do not take into account the potential use of SB-728-T, and are based on the assumption that eventually, patients with cell counts above 500 will begin to see declines). Further analysis of the data done by Sangamo showed that proliferation of SB-728-T post-infusion was "sustained over the year-long period reported in the study with median modified circulating cell numbers measured to be 2.04-fold relative to input at 7 days, 0.96-fold at 6 months and 1.15-fold at 1 year post-infusion." Sangamo's Phase I data suggests that SB-728-T has the potential to reshape the immune systems of HIV patients, and allow them to fight back against the disease.
On the company's Q3 conference call, Sangamo's executives were pressed about this data, and what it means for the way forward. Wedbush argued that the FDA may not deem the data strong enough for approval given that SB-728-T does not completely wipe out the HIV virus. Analyst Liana Moussatos asked, "Based on the Phase I data, I thought it was very interesting about the treatment resulting in kind of normalizing the immune system in HIV infected patients where the virus had destroyed part of it. Is this, I mean in your discussion with the FDA, if SB-728-T treatment just restored the immune system and, say, reduces the strange cancers that result or the infections but maybe doesn't reduce viral load to nothing. Would that still be a viable treatment that could get approved?" Sangamo CEO Edward Lanphier and Geoff Nichol, the company's Executive Vice President of Research & Development, responded that this is a "hotly contested kind of question," stating that in the HIV/AIDS market, FDA approval has historically been based on viral load, which is the focus of the company's Phase II trials for SB-728-T, and that the company's goal is to render HAART unnecessary by keeping CD4+ T-cell counts above 500.
While SB-728-T does not destroy the HIV virus in every single patient, it has shown promising results, and 2013 will offer investors 2 different catalysts that can drive shares of Sangamo higher. The market for HIV and AIDS treatments is set to reach nearly $22 billion by 2018, therefore Sangamo has a tremendous opportunity to create meaningful value for its investors, as well as offer HIV patients a new treatment option, even if it captures only a minority of the market.
Financials & Pipeline Review
Sangamo ended Q3 2012 with $75.816 million in cash & investments (per its latest 10-Q filing), and expects to end 2012 with around $75 million in cash & investments. Sangamo's operating cash burn for the first 3 quarters of 2012 totaled $8.305 million, and even if Sangamo were to burn $10 million per quarter, the company would still have nearly 2 years of capital left to finance its operations.
While Sangamo is, unsurprisingly, unprofitable, the company does generate revenue ($4.907 million in Q3 2012, up 164.24% year-over-year, and $12.723 million for the first 3 quarters of 2012, up 128.38% from the first 3 quarters of 2011). Sangamo has licensing agreements for its technology in place with Sigma-Aldrich (SIAL), Dow Chemical's (DOW) AgroScience division, and Shire (SHPG). The company's agreement with Sigma-Aldrich involves the use of ZFP technology as a research reagent, while its agreement with Dow involves the use of ZFP technology in plant research. Sangamo's agreement with Shire, struck at the beginning of 2012, is the most crucial of these 3 agreements. Under the terms, Shire paid Sangamo $13 million upfront and received an exclusive worldwide license to ZFP technology for 4 different genes, covering blood clotting Factors VII, VIII, IX, and X. Shire also received rights to 3 other gene targets. Of the 6 pipeline programs (including SB-728-T) that Sangamo is currently working on, Shire owns ZFP technology in Huntington's disease and hemophilia.
In October, Sangamo and Shire presented the first set of pre-clinical data for their experimental treatment for Huntington's disease. The disease is cause by a mutation in a person's HTT gene, which is inherited from their parents. The HTT gene is responsible for encoding a protein also known as HTT. The mutation consists of a repeated stretch of DNA, known as a "CAG repeat." Under normal circumstances, a person's HTT gene has 10-29 of these CAG repeats. Patients with Huntington's disease, however, usually have more than 39. The more CAG repeats, the earlier the onset of symptoms, which include muscle and nerve degeneration, and loss of memory and cognitive control. Patients usually die within 10-20 years of symptom onset. Preclinical testing in animals has shown that lowering levels of HTT protein can slow, and potentially reverse the progression of Huntington's disease. Sangamo's pre-clinical data showed that the production of mutated HTT messenger RNA fell by more than 90%, all while leaving normal HTT cells and RNA untouched. The company expects to see this program in clinical trials by 2015. The companies' hemophilia program is also set to move into clinical testing.
Options Strategy, Takeover Prospects, and Conclusions
For Sangamo investors, gains or losses in 2013 will be driven by new data regarding SB-728-T. Fortunately, Sangamo has listed options, and they allow more conservative investors to mitigate some risk while preserving upside potential (prices are accurate as of the close of trading on Monday, January 14).
Sangamo Options Strategies, August 17, 2013 Expiration Date
The August 17 expiration date is used because it encapsulates the first half of 2013, thereby allowing Sangamo investors to be protected through the company's first data release of SB-728-T, as well as giving them a chance to hold through the second data release. Of the options strategies listed above, the purchase of an $8 put is the most sensible. It caps losses at under 18% and requires a move of under 19% to be profitable, easily doable should Sangamo report positive data for SB-728-T. However, the 3 options strategies listed assume that investors have also purchased Sangamo stock outright. There is also another options strategy that I would like to highlight: the strangle. This strangle, also utilizing the August 17 options, involves purchasing the $2 call for $6.40 (giving an entry point of $8.40 per share, just 2.44% above where Sangamo is currently trading), as well as the $7 put for $0.95. The net cost is $7.35 per strangle, and it offers a similar level of protection as the options strategy outlined above, but with a smaller capital investment.
While there has been nothing substantial in the way of takeover rumors regarding Sangamo, there is always a possibility that a deal will occur given the company's cheap valuation in relation to the market that it targets. Just as GlaxoSmithKline (NYSE:GSK) did with Human Genome Sciences, Shire cuold move to take full control of Sangamo's pipeline. Alternatively, Gilead Sciences (NASDAQ:GILD) could strike a deal, although it would be motivated by different reasons. Gilead has delivered enormous profits to its investors by pioneering the treatment of HIV/AIDS; since going public in 1992, Gilead has returned almost 90,000% (Gilead has also helped enhance the lives of millions of patients). However, the fact that there is no cure for HIV/AIDS, and the fact that patients need continuous antiretroviral therapy to hold it at bay means that Gilead has much more stability than its biotechnology peers. However, should Sangamo and SB-728-T remove the need for such therapy, Gilead's business could be threatened. It is not unreasonable to assume that Gilead may make an offer Sangamo cannot refuse in order to protect its business. And with a market capitalization of less than $400 million, Sangamo is digestible for Gilead, even with a size-able premium. 2013 is poised to be a revolutionary year for Sangamo BioSciences, its investors, and HIV/AIDS patients.
(...)
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Dopo quest'annuncio la Sangamo ha fatto un bel salto in borsa,
http://www.gaininggreen.com/hot-biotech ... ks/122585/
C'è chi vocifera (www.poz.com) che quest'aumento potrebbe essere dovuto a tentativi di scalata in corso. Ma piuttosto io vedrei più probabile il fatto che solo adesso il mercato si sta rendendo conto delle potenzialità (finanziarie) della Sangamo Biosciences
http://www.medgadget.com/2013/01/new-te ... cells.html
Facendo pronostici rosei sul prossimo futuro:
http://seekingalpha.com/article/1112651 ... onary-2013
http://www.gaininggreen.com/hot-biotech ... ks/122585/
C'è chi vocifera (www.poz.com) che quest'aumento potrebbe essere dovuto a tentativi di scalata in corso. Ma piuttosto io vedrei più probabile il fatto che solo adesso il mercato si sta rendendo conto delle potenzialità (finanziarie) della Sangamo Biosciences
http://www.medgadget.com/2013/01/new-te ... cells.html
Facendo pronostici rosei sul prossimo futuro:
http://seekingalpha.com/article/1112651 ... onary-2013
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Sempre più ricerche in concorrenza con le ZFN di Sagamo. Questa è di Stanford (Matthew Porteus) e prevede sia la distruzione mediante nucleasi del gene che codifica per il CCR5, sia l'inserimento di tre geni resistenti all'HIV. La ricerca sta per partire sui modelli animali e, se tutto va bene, sarà in fase clinica fra 3-5 anni.
Articolo su Molecular Therapy (Nature) del 22 gennaio: non ancora online.
Comunicato stampa: Immune cells engineered in lab to resist HIV infection, Stanford study shows.
Articolo su Molecular Therapy (Nature) del 22 gennaio: non ancora online.
Comunicato stampa: Immune cells engineered in lab to resist HIV infection, Stanford study shows.
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
L'articolo uscito su thebodypro.com il 17 gennaio fa riferimento alla Sangamo.
La cosa che mi ha lasciato un pò di stucco è una (parziale?) notizia riportata al fondo dell'articolo in cui si dice che un sotto-studio condotto dalla Sangamo avrebbe rilevato la perdita nel tempo delle cellule modificate e un rebound virale in assenza di haart.
"As mentioned before, use of genetic engineering in the search for a cure for HIV has already had a head start. Another small biotech company, Sangamo Biosciences, has already done encouraging studies modifying CD4+ cells and infusing them in HIV-infected people. Sangamo has used zinc finger technology to modify CD4+ cells so that they contain an induced mutation of the CCR5 gene. In patients with undetectable HIV viral loads who remain on treatment, there has been a promising 7% uptake of those modified cells into the patient's immune system and gut mucosa. Some had normalization of their CD4+/CD8+ ratios, a measure of immune competence.
However, patients who stopped HIV medications in another sub-study using the same approach lost their gained modified cells with time and had viral rebound. Due to the length of the treatment interruption, we did not learn if such rebound reached a viral load set point lower than what patients had before starting HIV medications. Only one patient's immune system seemed to later control HIV. That patient was found to have been born with one mutation in his CCR5 receptor. Sangamo is now doing a study looking at this minority of people.
"
Mi sono perso qualcosa?

La cosa che mi ha lasciato un pò di stucco è una (parziale?) notizia riportata al fondo dell'articolo in cui si dice che un sotto-studio condotto dalla Sangamo avrebbe rilevato la perdita nel tempo delle cellule modificate e un rebound virale in assenza di haart.
"As mentioned before, use of genetic engineering in the search for a cure for HIV has already had a head start. Another small biotech company, Sangamo Biosciences, has already done encouraging studies modifying CD4+ cells and infusing them in HIV-infected people. Sangamo has used zinc finger technology to modify CD4+ cells so that they contain an induced mutation of the CCR5 gene. In patients with undetectable HIV viral loads who remain on treatment, there has been a promising 7% uptake of those modified cells into the patient's immune system and gut mucosa. Some had normalization of their CD4+/CD8+ ratios, a measure of immune competence.
However, patients who stopped HIV medications in another sub-study using the same approach lost their gained modified cells with time and had viral rebound. Due to the length of the treatment interruption, we did not learn if such rebound reached a viral load set point lower than what patients had before starting HIV medications. Only one patient's immune system seemed to later control HIV. That patient was found to have been born with one mutation in his CCR5 receptor. Sangamo is now doing a study looking at this minority of people.
"
Mi sono perso qualcosa?
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Sì, hai ragione: seguendo questa sperimentazione giorno per giorno, il rischio è proprio quello di andare in confusione e perdere qualche pezzo.cesar78 ha scritto:However, patients who stopped HIV medications in another sub-study using the same approach lost their gained modified cells with time and had viral rebound. Due to the length of the treatment interruption, we did not learn if such rebound reached a viral load set point lower than what patients had before starting HIV medications. Only one patient's immune system seemed to later control HIV. That patient was found to have been born with one mutation in his CCR5 receptor. Sangamo is now doing a study looking at this minority of people."
Mi sono perso qualcosa?
Siamo fermi, sostanzialmente, al CROI dell'anno scorso: i (pochissimi) pazienti che, dopo l'infusione di CD4 modificati, hanno sospeso la ART hanno tutti (tranne uno: il famoso "Trenton patient", eterozigota per la delezione Delta32), prima o poi ma comunque abbastanza in fretta, avuto un rebound delle viremie. Per quanto riguarda la perdita di CD4 modificati, è un risultato atteso, perché i linfociti T maturi non hanno la capacità proliferativa virtualmente infinita delle staminali e, dopo qualche divisione, inevitabilmente si esauriscono. Pertanto il numero di quelli modificati decresce e aumentano quelli prodotti dalle staminali, quindi CCR5+, aggredibili dal virus.
Ti riporto una sintesi dei risultati dell'ultimo CROI:
Dobbiamo dunque aspettare ancora un mese e mezzo per capire se i rumors sui successi di Sangamo hanno una qualche base di realtà o servono solo per far aumentare le sue quotazioni in borsa.Dora ha scritto:
- • La modificazione dei CD4 con l’SB-728-T continua a mostrarsi sicura e ben tollerata, gli eventi avversi di una certa entità sono stati rari e tutti reversibili.
• Si è visto un aumento dei CD4 in entrambi i gruppi, maggiore nei pazienti che partivano da un alto numero di CD4, minore negli altri, ma comunque sia consistente, sia persistente dopo un anno di follow-up. Analogo discorso sui CD4 modificati, che sono stati rilevati – in un caso - fino a 2 anni dopo il trattamento. Inoltre, le cellule sono andate a ripopolare la mucosa gastrointestinale.
• Il rapporto CD4:CD8 si è normalizzato negli immunologic non responders, mentre negli altri è aumentato.
• Si sono rilevati alti livelli di IL-2, IL-7 e IL-15 subito dopo l’infusione dei CD4, che potrebbero spiegare la rapidità dell’espansione dei CD4 trasfusi.
• Dopo la sospensione della HAART, si è avuto un iniziale rialzo delle viremie, seguito da un crollo (da 0,8 a 2 log) in 3 dei 6 pazienti. Al paziente che già di suo era eterozigote Delta32, la viremia è rimasta irrilevabile fino a quando – in base al protocollo – ha comunque dovuto riprendere la HAART.
• Prendendo come misura del reservoir i livelli di DNA provirale nei CD4, in 2 pazienti su 6, all’interruzione della terapia, si sono visti aumenti di 4 e di 9 volte. Tuttavia, questi aumenti si sono invertiti alla ripresa della HAART.
Re: [STUDI] Sangamo: CD4 e staminali resi CCR5- mediante ZFN
Potrebbe essere secondo te Dora solo un problema di "dosaggi"? Intendo la % di cd4 modificati troppo bassa (sicchè il ricambio non basta e si ha un rebound) 
