Un post su The Body ripreso dal Canadian AIDS Treatment Information Exchange offre molti interessanti dettagli sul CPG 7909, da cui anche si comprende perché questo agonista del TLR9 non sia già entrato nel protocollo di sperimentazione dell'eradicazione via panobinostat (e tante grazie alla Pfizer).Dora ha scritto:Seguendo una suggestione di Richard Jefferys, ho riletto la review di Rasmussen e Søgaard che ho citato all’inizio di questo thread – Eliminating the latent HIV reservoir by reactivation strategies – in particolare un paragrafo che avevo trascurato quando ho scritto il mio primo post, in cui si discute di immunoterapia e di agonisti del Toll-Like Receptor.
È possibile che questi ricercatori danesi vedano la via dell’eradicazione come una terapia combinata, formata da panobinostat per risvegliare l’HIV latente e CPG 7909, un agonista del TLR9 che dovrebbe contribuire a risolvere il “problema di Siliciano e Shang”.
In effetti, la stimolazione dell’immunità innata non-specifica attraverso il sistema dei TLR è utilizzata per trattare certe infezioni virali ed è anche una via che il gruppo di ricercatori dell’università di Aarhus sta sperimentando per migliorare l’immunizzazione (cfr. trial e articolo).
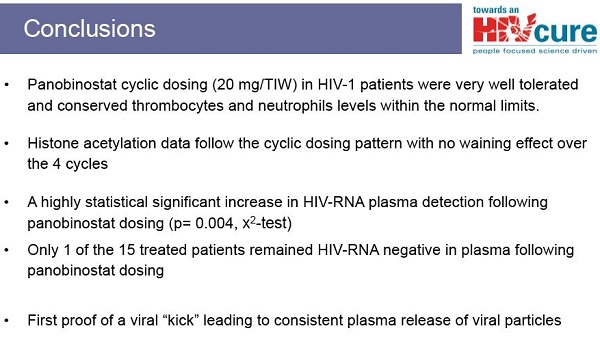
Da studi in vitro, Rasmussen e colleghi hanno visto che si crea un effetto sinergico se si combinano insieme degli HDACi con uno stimolante sintetico delle funzioni immunitarie che sfrutta il meccanismo di riattivazione dell’HIV via TLR9: trattando una linea di cellule latentemente infette con concentrazioni crescenti di questo stimolante sintetico (chiamato CpG ODN - CpG oligodeossinucleotidi e specificamente CPG 7909) e con basse concentrazioni di panobinostat, si sono visti aumenti rilevanti di produzione di HIV.
- As mentioned in our previous CATIE News story, Danish scientists have been studying potential ways of curing HIV infection with the experimental cancer drug panobinostat. Interim results of their clinical trial of this drug will be presented later this year at an international conference. In the meantime, Danish scientists have also been doing some other interesting cure research that may have gone underappreciated. This other research is the focus of this CATIE News story. Before getting into the details of their work, a bit of background is necessary.
A Lesson From History
In the late 1800s, New York surgeon William Coley began experiments to assess the impact of inducing bacterial infections on the course of certain cancers in people. Specifically, he deliberately caused serious skin infections with Streptococcus and later other bacteria. This idea was based on observation that some patients with cancer experienced unexpected remission of their disease when they also developed certain bacterial infections. However, the consequences of serious bacterial infections in the pre-antibiotic era could be dangerous. Eventually Dr. Coley and colleagues used injections of heat-killed bacteria (a mixture known as Coley's toxins). This resulted in limited success in carefully selected patients with certain cancers. Bear in mind that clinical trials in that era were not underpinned by rigorous statistics and they generally lacked a randomized, controlled design.
Side Effects
The initial effect of the therapy was to cause symptoms of a severe flu-like illness. Within an hour of injection of the vaccine, patients experienced severe but temporary chills, followed by a high fever. These and other symptoms cleared within 12 to 24 hours after injection. Some patients were given daily therapy with this vaccine, others received it every other day for weeks or months and then the administration was gradually reduced and ended, depending on the response of the cancer.
Researchers in the late 20th century suspected that these bacterial vaccines worked by stimulating the body to produce a mix of chemical messengers, called cytokines, which alerted the immune system to the presence of cancer. Furthermore, these cytokines also strengthened the ability of cells of the immune system to destroy some cancers.
Weakened Strains of Bacteria
After Dr. Coley's death, general interest in his bacterial vaccines waned. Today, some scientists are aware of the potential of Dr. Coley's work. Bacterial extracts are now used as part of the treatment for some cases of bladder cancer. In this instance, doctors use weakened strains of bacteria related to those that cause TB (tuberculosis). The strain of bacteria used is called BCG. This works by stimulating the immune system resident in the bladder and helping it attack this cancer. BCG is also used to make a vaccine to prevent TB. However, this vaccine is not highly effective at preventing TB and is seldom used in high-income countries today.
In the 1980s, Japanese researchers working with BCG found that exposing cells of the immune system to bacterial DNA improved their ability to attack cancerous tissue. In the 1990s, doctors in Japan conducted a clinical trial of bacterial DNA to treat patients with cancer. Their overall results were promising, with 43% of 75 patients demonstrating a response. However, due to the difficulty of creating uniform and safe concentrations of extracts of DNA derived from BCG, the Japanese Ministry of Health and Welfare rejected a request to license such therapy.
Pattern Recognition
In the mid-1990s, physician-investigator Arthur Krieg, M.D., and other scientists were trying to understand precisely how bacterial extracts, such as those used by Dr. Coley, could have such a profound impact on the immune system and cancer cells. In experiments in the lab, scientists exposed cells of the immune system to tiny pieces of nucleic acids (strips of DNA) arising from bacterial infections. This exposure to bacterial nucleic acids caused cells to produce a protective immune response. Further research found that a segment of the tiny pieces of nucleic acid were common to many bacteria and triggered a protective response by the immune system when it encountered different species of bacteria. This occurred because the immune system recognized a pattern in the nucleic acid of bacteria via specialized receptors. These receptors are called TLRs (toll-like receptors).
Recognizing Viruses and Cancer
TLRs act as part of the body's warning system for invasion by germs. Different TLRs serve to recognize different patterns in different types of germs. One type of TLR called TLR9 is particularly important. Researchers have found that TLR9 plays a role in helping the immune system sense viral infections such as HIV, HBV (hepatitis B virus) and HPV (human papilloma virus). All three viruses can cause varying degrees of immune dysfunction in people and have been associated with an increased risk for the development of different cancers. Furthermore, these particular viral infections weaken the ability of TLR9 to alert the immune system to the presence of viruses and tumours. Partly as a result of weakened TLR9 activity, and likely other immunologic dysfunction associated with these viral infections, the immune system is not able to contain and eradicate these viruses and the tumours they cause.
CpG -- A Refined Approach
As mentioned earlier, exposure to bacterial extracts such as Coley's toxins can cause unpleasant symptoms. A more refined approach to eliciting a protective immune response uses artificially created strings of DNA that mimic patterns found in bacterial and viral DNA. These artificial strings of nucleic acids are called CpG.
Researchers in several countries have tested the general safety and preliminary effectiveness of CpG 7909 (also known as agatolimod, PF-3512676, ProMune). This compound interacts with TLR9 and appears to boost its function, activating the immune system to better sense and fight viral infections and possibly some tumours. Several clinical trials have been done with CpG 7909 in HIV-negative patients with cancer. Several small clinical trials and two large phase III clinical trials (enrolling a total of about 1,600 patients with advanced lung cancer receiving chemotherapy) have been performed with CpG 7909 in HIV-negative patients. In these trials the drug appeared to be generally safe; however, there appeared to be limited anti-cancer effect.
CpG 7909 -- Tested in HIV-Positive Volunteers in Canada
In the past decade, a team of scientists at the Ottawa Hospital Research Institute tested CpG 7909 in HIV-positive people. In their experiments, the researchers used very small doses of this CpG together with a hepatitis B vaccine. The team found that this compound, when combined with the vaccine, increased the immune system's response to the vaccine and this response lasted a long time. The use of CpG 7909 was generally safe, with side effects (redness and swelling at the injection site) being temporary.
The New Danish Study
Several years ago, a team of researchers at Aarhus University in Denmark conducted a randomized, placebo-controlled study to compare the effect of a pneumonia vaccine with or without CpG 7909 in 97 HIV-positive people who were taking combination anti-HIV therapy (commonly called ART or HAART). In this study, repeated injections of 1 mg of CpG 7909 at the start and in the third and ninth months of the trial significantly increased the immune response to the vaccine.
However, the Danish team did some additional research. After the study was completed they reanalyzed stored blood samples from a subset of participants. The purpose of this reanalysis was to assess the impact that exposure to CpG 7909 may have had on the proportion of HIV-infected cells in the blood. The reason for this investigation was that laboratory studies done several years before with cells and HIV found that several CpGs had the ability to both stimulate HIV replication from resting infected cells and to interfere with HIV's ability to cause new infections.
The team found that the proportion of HIV-infected cells fell by 12% after each injection of CpG. This finding suggests that regular exposure to CpG 7909 can reduce the burden of HIV-infected cells in the body. This burden is called the "reservoir" by scientists and, in theory, a smaller reservoir should make curing HIV ultimately easier over the long term. However, separate clinical trials will be needed to assess this possibility, as none of the participants who received CpG injections were cured.
In contrast, among participants who received placebo, the proportion of HIV-infected cells remained about the same. Furthermore, participants who received injections of CpG appeared to have killer T-cells (called CD8+ cells) with increased anti-HIV activity. There was no detectable increase in HIV antibodies in participants.
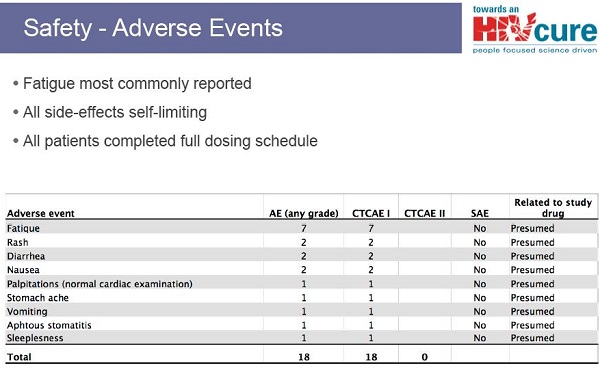
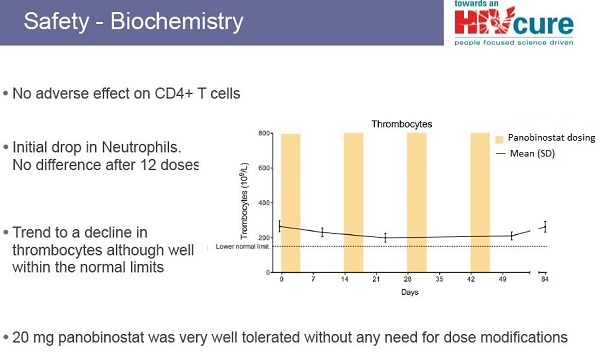
According to the researchers, CpG 7909 was "generally well tolerated," with mild side effects at the injection site (pain, swelling, redness, bruising). In some cases there were also temporary flu-like symptoms (such as fever, joint pain, chills and fatigue) and in 76% of participants, these were judged to be of "moderate to severe" intensity. Exposure to CpG did not reduce CD4+ cell counts. In some cases, there were very small and temporary increases in HIV viral load. But no participant had their viral load become detectable (that is, rise above the 50 copies/ml mark) for more than two consecutive blood tests. Further analyses found that CpG 7909 did not cause any toxicity to major organ-systems such as the bone marrow, liver or kidneys.
Caution Needed
The results from the Danish reanalysis are modest yet promising. However, they need to be interpreted cautiously, at least for the following reasons:- - Reanalyzing data from a study designed for a different purpose can, at best, yield interesting results. However, such reanalysis cannot produce definitive results. The findings from the Danish study can be used to design a clinical trial to assess the impact of CpG 7909 on changes in the proportion of HIV-infected cells, perhaps with more participants and over a longer period.
- The reanalysis had additional gaps: The study was not formally designed to assess the impact on the immune system's ability to detect and destroy HIV-infected cells. Therefore, the scientists are not certain how repeated exposure to CpG 7909 apparently reduced the burden of HIV-infected cells.
What is clear is that no one has yet been cured of HIV by repeated exposure to CpG 7909 over a period of 10 months. Also, such a cure -- with this or any other therapy -- is not imminent. Rather, much more study is required of this exciting compound, perhaps in combination with other experimental drugs in HIV-positive people who are taking ART.
Problems With Access to CpG 7909
CpG 7909 was developed by the Coley Pharmaceutical Group, which was then acquired by the pharmaceutical company Pfizer in 2011, along with all of the rights for commercially testing and using CpG 7909 in people. Pfizer is developing a new CpG molecule that recently entered clinical trials, but they have not been providing this to outside groups for testing. CpG 7909 is in cancer vaccines being developed by GlaxoSmithKline as well as in several other cancer vaccines being developed by university-based scientists. Although clinical trials with CpG 7909 are ongoing in HIV-negative people, Pfizer has not continued Coley's policy of providing the compound to academic researchers for their research. Until this situation changes, it is not likely that CpG 7909 will be tested in clinical trials in HIV-positive people.
Not Giving Up
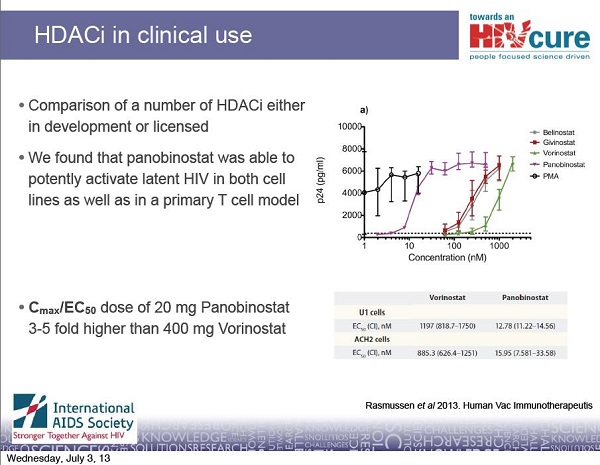
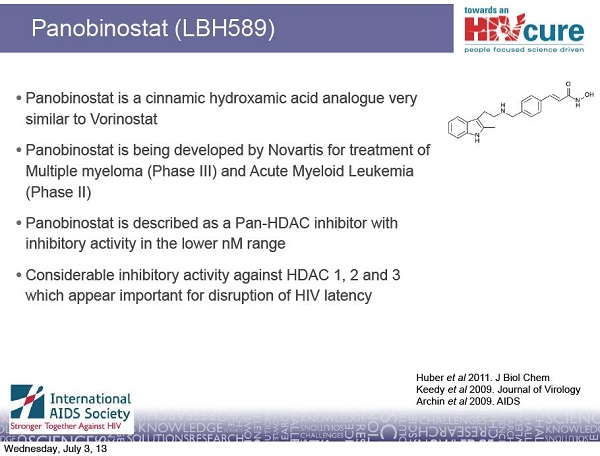
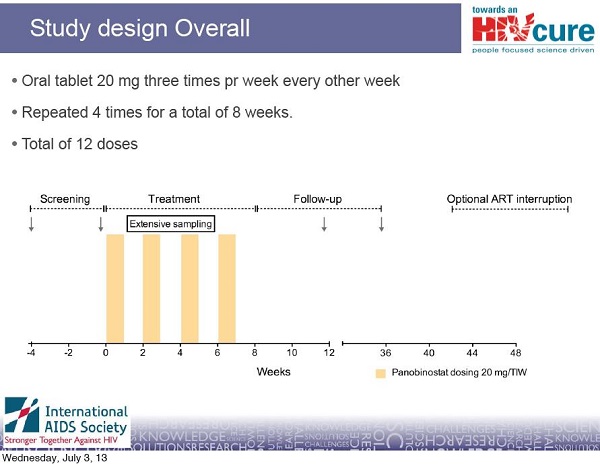
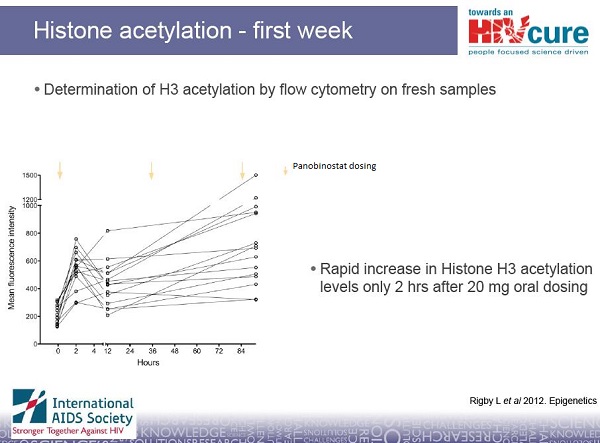
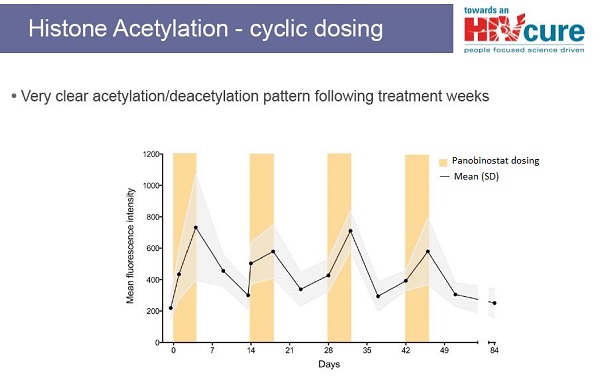
Although the Danish scientists cannot currently access CpGs for clinical trials in people, they are not giving up on cure research. They have at least another approach to a potential cure for HIV. In collaboration with other scientists in Australia, Sweden and the United States, they are testing a drug that will hopefully help to drive HIV out of hiding. This approach has the potential to reduce the burden of HIV-infected cells in the body and make future attempts at a cure perhaps easier. The drug being tested is an experimental cancer therapy called panobinostat, made by the pharmaceutical company Novartis. Panobinostat belongs to a class of drugs called HDAC inhibitors. Interim results from their study of panobinostat will be released later this year. To find out more about HDAC inhibitors and their potential and challenges for curing HIV, see TreatmentUpdate 196.
Cure Research in Context
The journey toward a cure will not be easy and many challenges lie ahead. Some of the challenges are known, others may only become known as experiments proceed. As with any great scientific endeavour, there will be setbacks. The initial wave of cure research experiments over the next five to 10 years should be viewed as exploratory and their results highly preliminary. This research will seek to answer important scientific questions that can then be used to build a foundation toward a cure. In the meantime, research funding agencies need to show patience and sustained funding as hardworking scientists struggle against the many challenges that lie in their path as they search for avenues to explore in the quest for an HIV cure.
Acknowledgement
We thank Ole Søgaard, M.D., and colleagues at Aarhus University, Denmark, and Arthur Krieg, M.D., RaNA Therapeutics, Cambridge, Massachusetts, for their helpful comments, research assistance and expert review.
References
- 1- Coley RB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clinical Orthopaedics and Related Research. 1991 Jan;(262):3-11.
2- Starnes CO. Coley's toxins in perspective. Nature. 1992 May 7;357(6373):11-2.
3- Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995 Apr 6;374(6522):546-9.
4- Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Japanese Journal of Infectious Diseases. 1999 Feb;52(1):1-11.
5- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20:197-216.
6- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annual Review of Immunology. 2002;20:709-60.
7- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335-76.
8- Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nature Medicine. 2003 Jul;9(7):831-5.
9- Hirsch I, Caux C, Hasan U, et al. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends in Immunology. 2010 Oct;31(10):391-7.
10- Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005 Sep 23;19(14):1473-9.
11- Søgaard OS, Lohse N, Harboe ZB, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clinical Infectious Diseases. 2010 Jul 1;51(1):42-50.
12- Winckelmann AA, Munk-Petersen LV, Rasmussen TA, et al. Administration of a Toll-Like Receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013 Apr 26;8(4):e62074.
13 Shan L, Siliciano RF. From reactivation of latent HIV-1 to elimination of the latent reservoir: the presence of multiple barriers to viral eradication. Bioessays. 2013 Apr 24.
14- Aarhus University Hospital. Correction to HIV story. Press release. 3 May 2013.
15- Aarhus University Hospital. Danish scientists get one step closer to a cure. Press release. 1 May 2013.
16- Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Therapeutics. 2012 Apr;22(2):77-89.
17- Rasmussen TA, Tolstrup M, Winckelmann A, et al. Eliminating the latent HIV reservoir by reactivation strategies: Advancing to clinical trials. Human Vaccines & Immunotherapeutics. 2013 Apr 1;9(4).
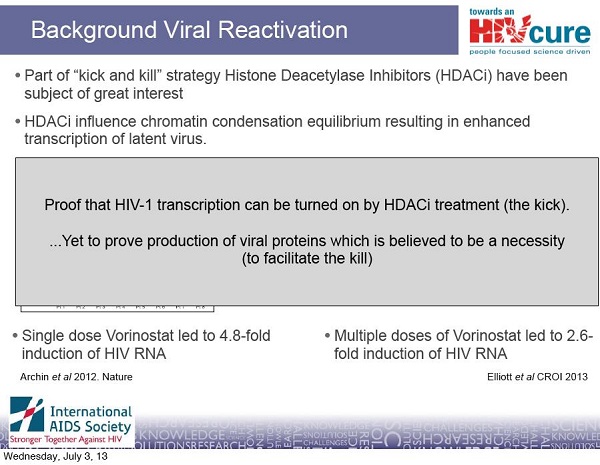
18- Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012 Jul 25;487(7408):482-5.
19- Deeks SG. HIV: Shock and kill. Nature. 2012 Jul 25;487(7408):439-40.
20- Cillo A, Sobolewski M, Coffin J, et al. Only a small fraction of HIV-1 proviruses in resting CD4+ T cells can be induced to produce virions ex vivo with anti-CD3/CD28 or vorinostat. In: Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections, 3-6 March 2013, Atlanta, U.S. Abstract 371.
21- Kent SJ, Reece JC, Petravic J, et al. The search for an HIV cure: tackling latent infection. Lancet Infectious Diseases. 2013; in press.
22- Katlama C, Deeks SG, Autran B, et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013; in press.
23- Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: A study of the Berlin patient. PloS Pathogens. 2013 May;9(5):e1003347.
24- Buitendijk M, Eszterhas SK, Howell AL. A Toll-Like Receptor-7 agonist that inhibits HIV type 1 infection of human macrophages and activated T cells. AIDS Research and Human Retroviruses. 2013 Jun;29(6):907-18
- - Reanalyzing data from a study designed for a different purpose can, at best, yield interesting results. However, such reanalysis cannot produce definitive results. The findings from the Danish study can be used to design a clinical trial to assess the impact of CpG 7909 on changes in the proportion of HIV-infected cells, perhaps with more participants and over a longer period.