Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection

Dall'inizio è stato studiato come farmaco long acting, somministrato per via sottocutanea.giovane888 ha scritto: ↑lunedì 27 giugno 2022, 13:41che poi dai risultati, non ho capito se per persone normali verrà approvato del tipo: dolutegravir e iniezione semestrale o orale.
Cioè oltre agli usi iniettivi, essendo di un'altra classe, verrà approvata come classe di farmaci orali?
Una nuova duplice terapia, immagino dolutegravir e lenacapavir, Lenacapavir + emtricitabina e tenofovir alafenamide o lenacapavir + lamivudina e tenofovir disoproxil fumarato.
Immagino siano in corso duplici o triplici...
BACKGROUND: Lenacapavir (LEN), a potent first-in-class capsid inhibitor, is in development as a 6-monthly subcutaneous (SC) injection for treatment and prevention of HIV-1 infection. In animals, SC injection led to reversible chronic granulomatous inflammation at the injection site as a foreign body response to LEN drug depot.
METHODS: We characterized the injection site reactions (ISRs) in participants who received at least one dose of subcutaneous (SC) LEN 927 mg (2 x 1.5 mL) in clinical studies in heavily treatment experienced (CAPELLA) and in treatment naïve (CALIBRATE) people with HIV (PWH).
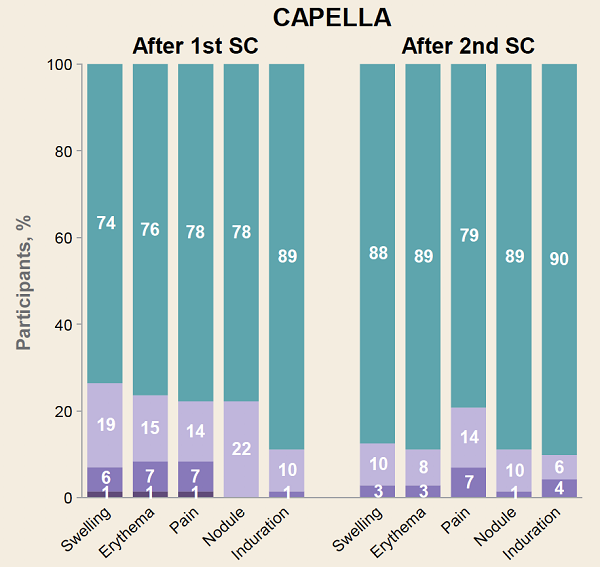
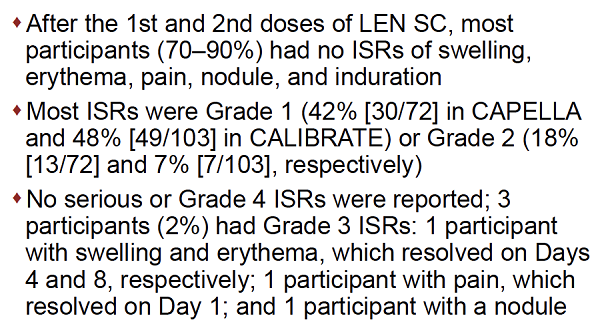
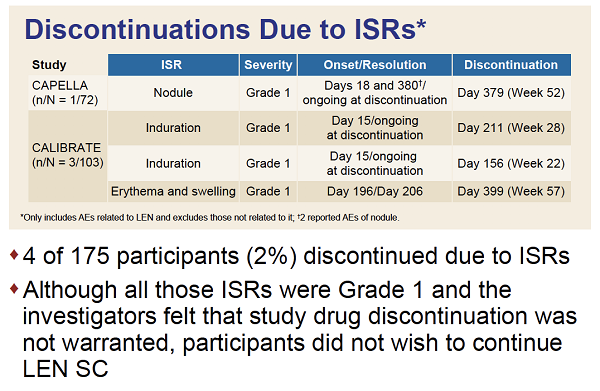
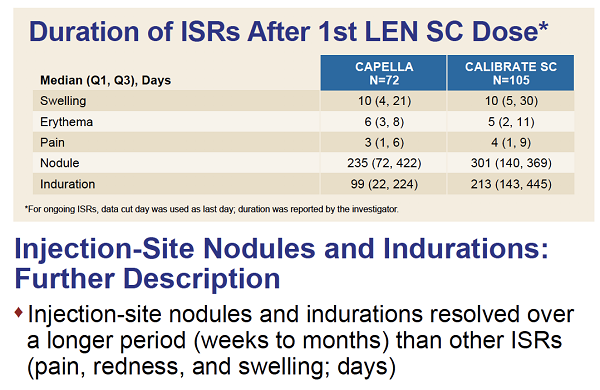
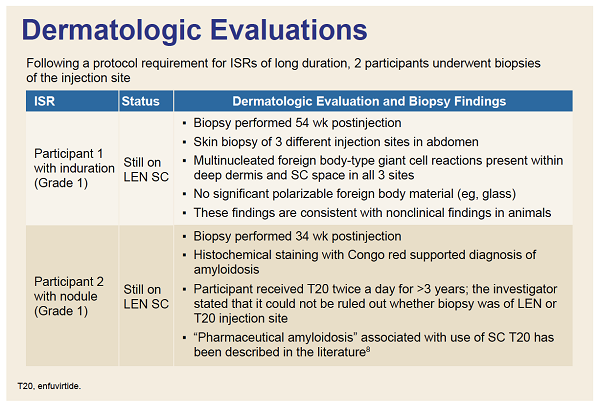
RESULTS: In CAPELLA, 72 participants received at least one and 70 two doses of SC LEN. In CALIBRATE, 103 received at least one and 95 two doses. The median duration of follow up was 376and 449 days, respectively. Most ISRs (97%, 99/102 participants) were Grade 1 or 2, rarely leading to discontinuation (4 participants: 1 [nodule], 2 [induration] 1 [erythema/swelling]). Common ISRs (any ISR '¥10% in both studies) were swelling, erythema, pain, nodule and induration, which generally occurred less frequently with subsequent injection (Table). Swelling, erythema and pain generally resolved within days; nodule and induration resolved over months. Investigators reported nodules and induration as generally palpable, not visible, non-erythematous, nonpainful.
CONCLUSIONS: Among PWH using SC LEN, ISRs were generally mild to moderate, rarely leading to discontinuation, and decreased in frequency with subsequent injection. Most ISRs resolved within days, while induration and nodules gradually improved over months but were generally not visible or painful. The pattern and nature of ISRs is consistent with preclinical experience.